Leveraging AI for Multi-Omics Analysis and Precision Medicine in Non-Small-Cell Lung Cancer NSCLC: Opportunities and Challenges
The Role of AI in Multi-Omics Analysis for NSCLC Treatment:
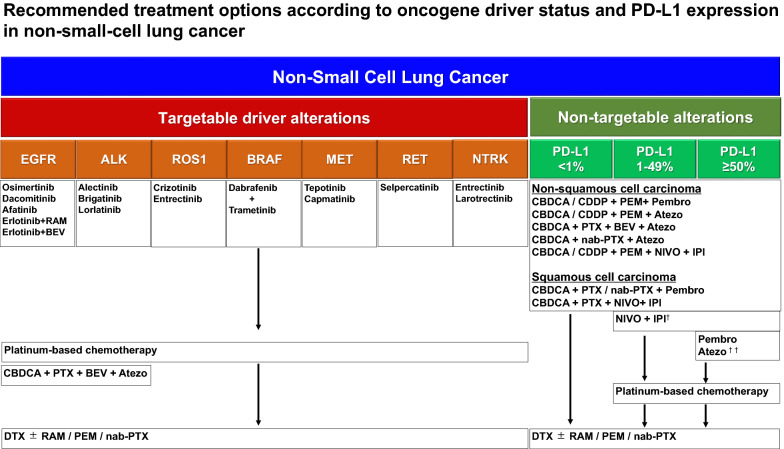
The integrated multi-omics data analysis—including genomic, transcriptomic, proteomic, metabolomic, and interactomic data—has become essential for understanding the complex mechanisms behind cancer development and progression. While advancements in multi-omics have revealed crucial insights into cancer, particularly in non-small-cell lung cancer (NSCLC), the analysis of this data remains highly labor-intensive. To address this, AI technologies, especially machine learning and deep learning, are being increasingly employed to streamline the process. AI systems can efficiently process large datasets, identifying patterns and biomarkers that may be overlooked in traditional methods. This leads to the development of more precise predictive models for personalized treatments, such as immunotherapy and targeted therapies.
Recent progress in AI-driven analysis has demonstrated its potential to transform cancer research and treatment strategies. By integrating AI with multi-omics data and clinical information, researchers can create comprehensive models that help in early cancer detection, prognosis prediction, and evaluation of treatment efficacy. AI-based models are particularly useful for NSCLC, where identifying druggable mutations and immune checkpoints has paved the way for tailored therapies. However, resistance to treatments remains a significant challenge, highlighting the need for AI to assist in predicting treatment responses and side effects. AI is expected to play a critical role in advancing personalized medicine and improving treatment outcomes for NSCLC patients.
AI in Medicine: Concepts and Applications:
AI in medicine can be categorized into rule-based and machine-learning approaches. Rule-based AI follows predefined instructions to reach solutions, effective in simple situations but limited in complexity. Machine learning generates rules from data patterns, including supervised, unsupervised, and reinforcement learning. Supervised learning is commonly used for medical image classification but requires labeled data, while unsupervised learning identifies patterns without labeled inputs. Deep learning uses neural networks to analyze medical images and improve diagnostics, such as identifying prostate cancer features from histopathology images.
AI Applications in Omics Data and Clinical Information Analysis:
AI, particularly machine learning, plays a vital role in analyzing omics data and clinical information, enabling physicians to predict health trajectories from vast datasets. Deep learning, which requires large datasets, is commonly applied, though machine learning models are often favored due to the limited availability of omics data. Techniques like LASSO regression and PCA help narrow features, while supervised models like SVM and random forest aid in classification and prediction tasks, including disease severity and mortality rates.
Advancements in AI and Omics Data for Early Detection of NSCLC:
NSCLC is often diagnosed at a late stage, where survival outcomes are poor. Early detection significantly improves prognosis, but current screening methods, such as low-dose CT (LD-CT), have limitations due to high costs, false positives, and the exclusion of younger non-smokers. AI-based diagnostic systems, like computer-aided detection (CADe) and computer-aided diagnosis (CADx), are emerging to assist radiologists in identifying early-stage lung nodules. While small sample sizes and unvalidated models have constrained their broader clinical adoption, recent collaborations have demonstrated promising results. Notable advancements include Optellum’s Lung Cancer Prediction CNN, which has shown superior performance over existing models, and a deep-learning model developed by Google and Northwestern University that achieved 94% accuracy in detecting malignant lung nodules.
Integrating AI with omics data also advances biomarker discovery to complement LD-CT screening and reduce false positives. New technologies, such as mass spectrometry, enable the detection of proteins associated with early-stage lung cancer, like surfactant protein B (pro-SFTPB). ML models have further enhanced biomarker identification, as demonstrated by lipidomic and RNA biomarker studies that achieved high accuracy in detecting NSCLC. The future of NSCLC detection lies in integrating AI with imaging diagnostics and omics data, offering improved early detection and insight into lung cancer’s molecular mechanisms.
AI and Molecular Targeted Therapy in NSCLC: Future Directions and Challenges:
Advancements in AI are poised to enhance the discovery of selective inhibitors for NSCLC with druggable mutations, improving treatment precision. AI facilitates the virtual screening of compounds and predicts clinical trial outcomes, crucial for overcoming drug resistance and optimizing targeted therapies. However, challenges remain, such as high development costs, resistance mechanisms, and ethical concerns over data privacy in omics research. Collaborations between academia and industry and AI’s ability to analyze vast datasets promise to refine treatment strategies and patient selection, improving NSCLC outcomes.
Check out the Paper. All credit for this research goes to the researchers of this project. Also, don’t forget to follow us on Twitter and join our Telegram Channel and LinkedIn Group. If you like our work, you will love our newsletter..
Don’t Forget to join our 52k+ ML SubReddit
Sana Hassan, a consulting intern at Marktechpost and dual-degree student at IIT Madras, is passionate about applying technology and AI to address real-world challenges. With a keen interest in solving practical problems, he brings a fresh perspective to the intersection of AI and real-life solutions.









